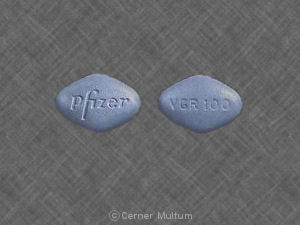
Pfizer VGR 100
Generic Name: sildenafil
This pill with imprint “Pfizer VGR 100” is Blue, Four-sided and has been identified as Viagra 100 mg. It is manufactured by Pfizer U.S. Pharmaceuticals Group.
Viagra is used in the treatment of erectile dysfunction; sexual dysfunction, ssri induced and belongs to the drug class impotence agents. There is no proven risk in humans during pregnancy. Viagra 100 mg is not a controlled substance under the Controlled Substance Act (CSA).
What are the possible side effects of oral sildenafil (Revatio, Viagra)?
Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
During sexual activity, if you become dizzy or nauseated, or have pain, numbness, or tingling in your chest, arms, neck, or jaw, stop and call your doctor right away. You could be having a serious side effect of sildenafil.
Stop using sildenafil and call your doctor at once if you have a serious side effect such as:
- sudden vision loss;
- ringing in your ears, or sudden hearing loss;
- chest pain or heavy feeling, pain spreading to the arm or shoulder, nausea, sweating, general ill feeling;
- irregular heartbeat;
- swelling in your hands, ankles, or feet;
- shortness of breath;
- vision changes;
- feeling light-headed, fainting; or
- penis erection that is painful or lasts 4 hours or longer.
Less serious side effects may include:
- warmth or redness in your face, neck, or chest;
- stuffy nose;
- headache;
- memory problems;
- upset stomach; or
- back pain.
- Generic Name:
- sildenafil
- Imprint:
- Pfizer VGR 100
- Strength:
- 100 mg
- Color:
- Blue
- Size:
- 14.00 mm
- Shape:
- Four-sided
- Availability:
- Prescription only
- Drug Class:
- Impotence agents
- Pregnancy Category:
- B – No proven risk in humans
- CSA Schedule:
- Not a controlled drug
- Manufacturer:
- Pfizer U.S. Pharmaceuticals Group
- National Drug Code (NDC):
- 00069-4220
-
Other Manufacturers / Repackagers:
| NDC Code | Manufacturer / Repackager |
|---|---|
| 54569-4570 | A-S Medication Solutions, LLC (repackager) |
| 66116-0205 | Medvantx Inc (repackager) |
| 55289-0524 | PDRX Pharmaceuticals Inc (repackager) |
| 54868-4706 | Physicians Total Care Inc (repackager) |
| 66267-0406 | Nucare Pharmaceuticals Inc (repackager) |
| 49999-0316 | Lake Erie Medical and Surgical Supply (repackager) |
| 63874-0481 | Altura Pharmaceuticals Inc (repackager) |
| 67544-0356 | Prepak Systems Inc (repackager) |
| 33358-0356 | CorePharma, LLC (repackager) |


Recent Comments